It's density, buoyancy and compression.
I love this project so much my ninth grade students and I made a giant (6' tall) Cartesian diver exhibit for our local children's museum. Of course, there weren't any soda bottles that big. We fabricated it using a rigid Plexiglas tube. For pressure, we pumped in air with a bicycle pump. It worked great, and we had fun.
When I do this project with groups of kids, I ask what happens if a stone drops in water. Every kid knows that it sinks. And they all know what happens when a piece of foam is dropped in water. Then I ask what would happen if one ton of foam were dropped into the ocean. Here there is less certainty, and it gets to the heart of what density is all about. Density isn't just about weight. It is just as much about how much space something takes up. Density is the ratio of weight divided by volume. If the density is greater than water, it sinks. If the density is less than water, it is buoyant.
I get a thrill out of seeing air compress. My kids say I'm easily amused, but after many years of knowing in theory that air was compressed into scuba tanks it was exciting to see it happen with my own eyes.
Here I will stop and refer you to a great web page about Cartesian divers. It is by a science teacher who not only did a phenomenal job of researching the history on the device, but who provides a great example of how to teach. Read about things like how student simulated a zero-gravity environment (jumping off a diving board into a pool) to test a theory about how the diver works!
Cool Variations of Divers
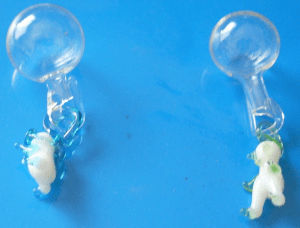
Here is a picture of beautiful hand-blown glass divers sent to me by Eugenia from Mexico, along with an explanation:
When I found about the Cartesian Divers, I remembered mine. But these are made of blown glass. I'm sending you 2 pictures. These I bought in Puebla, Mex. 20 years ago. I remember my father putting one of his in a white wine bottle and pushing the cork in so the diver would go down, and he controlled the depth with the cork. My brothers and me were little children and it was like magic. (There were no recyclable PET bottles to use). So my father's divers must had been 50 years old or so. We called them "tiny divers". My father was an accountant so didn't explain the science part, but I remember him as a science man, he had a lot of nice things, like the drinking bird (Photo) -(I would love to hear your explanation of how that works) and many amazing science toys.
Now I'm teaching my 9 years old son about the wonderful science things that happens around us and your page makes it easier and fun.
Professor E (aka Paul Eisenzimmer) passed on a great tip. You can replace the lid of the diver bottle with a "fizz keeper," which are available at grocery stores. Ostensibly these tiny air pumps that screw on are supposed to create high pressure inside the bottle so less carbon dioxide fizzes out of solution. However, we know that their highest calling is to pressurize Cartesian divers!
And here is an amazing tip from Eric Knispel, a science teacher at the John Burroughs School in Montana:
"Hi Slater, Wanted to share a favorite demo with you about Divers. After my 10th graders make their own as a lab activity using plastic pipettes (I ask them for 3 different colors diving in a specific order-blue, yellow, no color) I bring out my glass bottle diver."
"Using a glass bottle with flat sides (Italian dressing or whiskey bottle) I fill it completely full of water and add a diver that just barely floats. Squeezing the glass sides will give just enough pressure to flex the glass and sink the diver. It is very sensitive to temperature so on the days I find it already sunk, I squeeze the NARROW sides of the bottle and make the diver float again. (flexing the bottle into an oval increases the volume and reduces the pressure on the diver allowing it to float). The students don't believe it at first, assuming it is the heat from my hands etc."
"The divers are made from disposable pipettes, cut the stem off, glue on a few small metal nuts from the hardware store, fill with water until just floating, seal the ends with glue to keep colored water inside or leave open if no color is desired."
"PS: Mustard and ketchup packets from fast food places are the simplest and easiest divers I have ever come across. Put packet in plastic bottle of water and squeeze!" (editor's note: this is true as long as there is a little air trapped in the packet)
You can see a beautiful activity related to density that Eric has his students do if you click here. It is layers of different concentrations of saline solution (salt and water mixture). Because each concentration is differently colored, it forms a work of art!
And here is spinning variation from engineer and dad Harry Shuttleworth of the U.K. Hoping to have a picture of it soon. I've tried it and it works beautifully! I wish I'd thought of the idea.
"A few years ago I made some cartesian divers for the kids, similar to those also on your site. I also used 2l pet bottles, but I made the diver from the nozzle from a gunge gun (silicone etc), with the tip blocked, and weighted down with a 8mm nut and bolt. To get the effect of a wizards head under the cone "hat" I had dipped the bolt in a bath of hot melt glue to get a nice blob (and drew a face on). I then put small holes through the side of the hat to allow the water in."
"For additional fun, on one of them I set up the holes to be tangential, so it would spin as it pushed the water out when rising."
I'd like to know how this project goes for you. I'm happy to answer questions about it. Feedback from you is an important way for me to know what works and what needs clarification.
